MENU
FR | EUR
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Service pour pipette
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
-
-
-
-
-
- Services pour bioprocédés
- Services pour centrifugeuse et rotors
- Service pour pipette
- Services pour Mastercycler
- Services pour automates de pipetage
- Services pour congélateurs
- Services pour incubateurs
- Services pour agitateurs
- Services pour appareils de photométrie
- Service de contrôle de la température et de l’agitation
-
FR | EUR
-
- Centrifugeuses de paillasse
- Centrifugeuses au sol
- Centrifugeuses réfrigérées
- Microcentrifugeuses
- Centrifugeuses multi-fonctions
- Centrifugeuses haute vitesse
- Ultracentrifugeuses
- Concentrateur
- High-Speed and Ultracentrifugation Consumables
- Accessoires
- Tubes
- Plaques
- Gestion des appareils
- Gestion des échantillons et des informations
- Produits IVD
Aucun résultat trouvé
Chercher des suggestions
Perfusion – Making the Most out of Your Working Volume
Lab Academy
- Bioprocédés
- Microbiologie
- Biologie cellulaire
- Culture cellulaire
- Culture de microorganismes
- Modularité
- Bioprocédés
- Test
Bioprocessing of eukaryotic cells and microorganisms is used in the production of a wide range of goods, including biopharmaceuticals for diagnostic and therapeutic purposes, food and feed supplements, biofuels, and chemical building blocks. The addition of nutrients will affect the cell density and viability, the process duration, and as a result, the product titer, yield, and cost. This is why optimizing the nutrient supply is key in bioprocess development.
Of nutrients and by-products—ways to control the culture environment
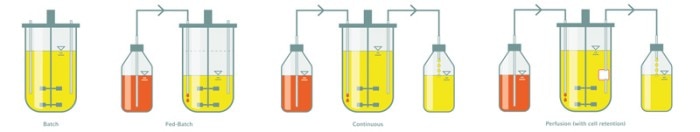
Despite the great variety of organisms, process parameters, and desired end-products, all bioprocesses have certain essential commonalities: The growth medium is inoculated with the desired cell line or microbial strain, which under favorable process conditions multiplies, consumes nutrients, and produces the desired end-product as well as by-products like lactate and ammonium.In detail this looks different depending on the process mode.
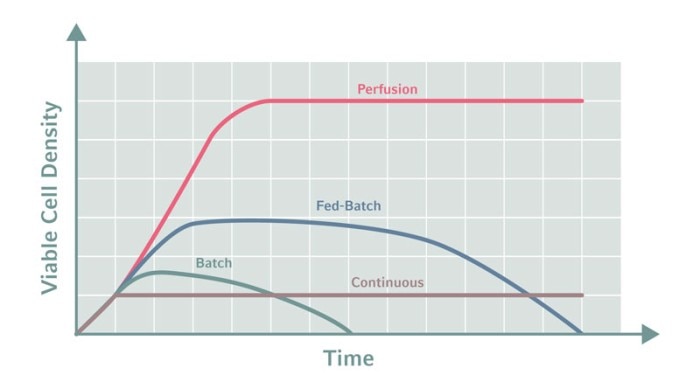
Batch and fed-batch bioprocesses are well known and widely used. In a batch process the culture grows in the initially supplied batch of medium. The product accumulates in the culture. At some point the cells will stop growing and the proportion of viable cells will decline, because nutrients are consumed and toxic metabolites get concentrated. The fed-batch process is a variation of the batch process. Here the culture is fed to keep the concentration of nutrients constant.
High substrate concentrations can be achieved, resulting in higher cell densities and product titers, and prolonged cell viability, but toxic metabolites still accumulate over time. For a couple of years now, continuous cultivation has been discussed as a potential game changer in biopharma production. The idea is to keep the culture conditions constant by constantly adding fresh medium and harvesting ‘used’ medium.
Lire moins
Continuous culture and perfusion—two sides of the same coin
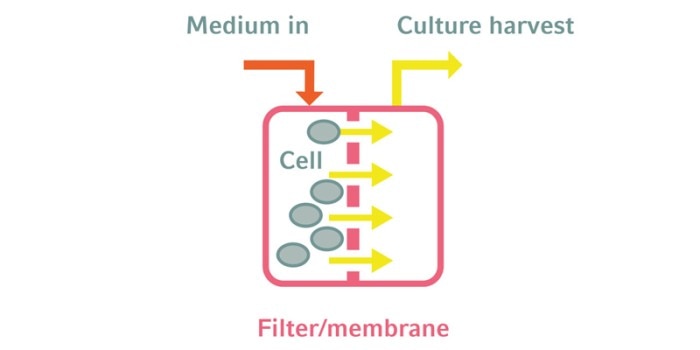
The terms perfusion and continuous cultivation describe two culture modes which are similar, though not the same [1]. In both modes the cell environment is kept constant by continuous exchange of the culture medium. The fundamental difference between them lies in the development of the cell density. In a continuous culture a portion of the mixed culture broth (medium + cells) is harvested, which results in a constant cell density. In a perfusion culture only the medium is harvested and the cells are kept in the culture by a cell retention device. The advantages are obvious: Consumed nutrients are replaced and toxic metabolites are removed from the culture―without diluting the cell density. As a result growth rate and cell viability stay high, in principle over many weeks.Continuous cultures are mainly used in R&D applications that require a constant culture environment. One example is the determination of an organism’s growth rate. In continuous mode the culture is diluted at a constant rate. Under steady-state conditions the dilution rate equals the specific growth rate and the cell density stays constant. This experimental setup can also be used to select for fast-growing clones, which can be interesting producer organisms. In a continuous culture, fast-growing mutants will overgrow their slower-growing siblings over time.
Lire moins
A potential game changer—perfusion in biopharma production
Perfusion promises great cost savings, so it is not surprising that it has received so much attention. Perfusion processes can deliver more product for a given bioreactor volume as they can maintain higher viable cell densities than traditional fed-batch processes. Smaller bioreactors can be used, saving precious lab space. And perfusion processes offer more flexibility in the scale of production, making, for example, large amounts of blockbuster drugs, smaller quantities of orphan drugs or less stable proteins, and responding to fluctuations in the market. Scale can be varied by modulating the process time or by changing the number of parallel production lines. This can be easier than changing the volume of a traditional fed-batch process.Product quality can also benefit; the product is constantly harvested in a perfusion process and thus the duration of its stay in the culture broth is cut short. This reduces the risk of degradation and aggregation, which is particularly beneficial in the manufacturing of less stable proteins. Furthermore, it has been reported that the constant conditions in a perfusion process improve the consistency of certain parameters, like the protein glycosylation profile for instance. Recent studies indicate that perfusion also works for cultivation of stem cells for research and cell-based therapy [2].
Hold them back
Key to the perfusion process is the retention of cells in the culture, using one of several approaches:1. Filtration: Filters that hold back the cells and let the liquid and small molecules pass are widely used. The simplest variation is a spin filterin the bioreactor with a dip tube inside to allow the harvest of cell-free medium. Spin filters are relatively cheap, but have some limitations like the tendency to clog. Tangential flow filtration (TFF) and alternating tangential flow (ATF) filtration devices are frequently used. In TFF the liquid flows past the pores of hollow fiber filters tangentially, rather than being forced through them orthogonally, thus reducing the likelihood of clogging. ATF devices use the same principle of tangential flow but reverse the direction of flow once in each cycle to minimize fouling and further reduce shear forces on the cells.
Lire moins
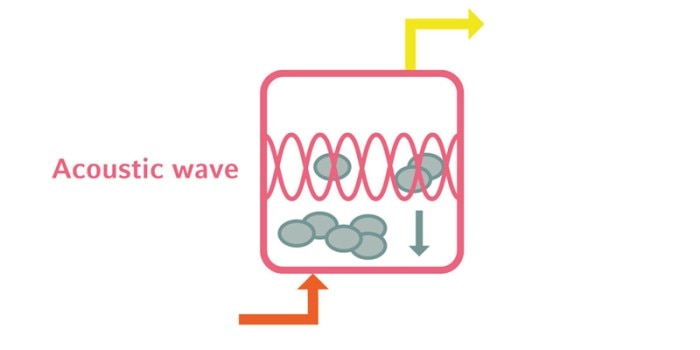
2. Acoustic wave separation: Acoustic wave separation is based on the formation of a three-dimensional standing wave in the liquid. Cells are trapped and aggregate at the nodes of the wave. The cell suspension is then cleared as the aggregates sediment.
Lire moins
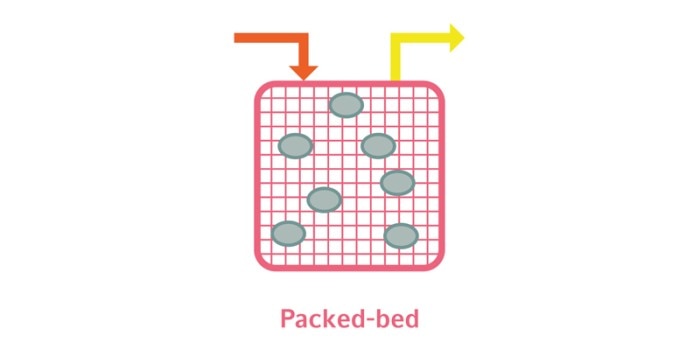
3. Packed-bed vessel: Packed-bed bioreactors are filled with a solid growth support on or in which the cells are immobilized. One such support is Fibra-Cel® discs. Fibra-Cel is a porous meshwork of polyester and polypropylene that provides a growth surface for adherent cells and entraps suspended cells. Immobilization of cells in the matrix facilitates the easy harvest of cell-free medium from the bioreactor.
Lire moins
In summary
Perfusion offers great advantages. Before deciding on the right cell retention method, several attributes need to be considered, including costs, the shear forces the cells encounter, how prone the devices are to clogging, and the cell densities that can be achieved.There is no general answer to the question, which process mode is the most cost-effective, but it has to be asked for any given biopharmaceutical. Costs for process development, medium, bioprocess equipment, and lab-space have to be taken into account. Perfusion is on the rise. Especially when planning a new process or even a whole new bioprocess facility it is for sure worth taking a very close look at it.
References:
[1] Konstaninov K, Cooney C (2014): White Paper on Continuous Bioprocessing. May 20–21, 2014 Continuous Manufacturing Symposium. Wiley Online Library. DOI 10.1002/jps.24268
[2] Kropp C, Kempf H, Halloin C, Robles-Diaz D, Franke A, Scheper T, Kinast K, Knorpp T, Joos TO, Haverich A, Martin U, Zweigerdt R, and Olmer R (2016): Impact of Feeding Strategies on the Scalable Expansion of Human Pluripotent Stem Cells in Single-Use Stirred Tank Bioreactors. Stem Cells Transl Med 5 10:1289.
Lire moins

